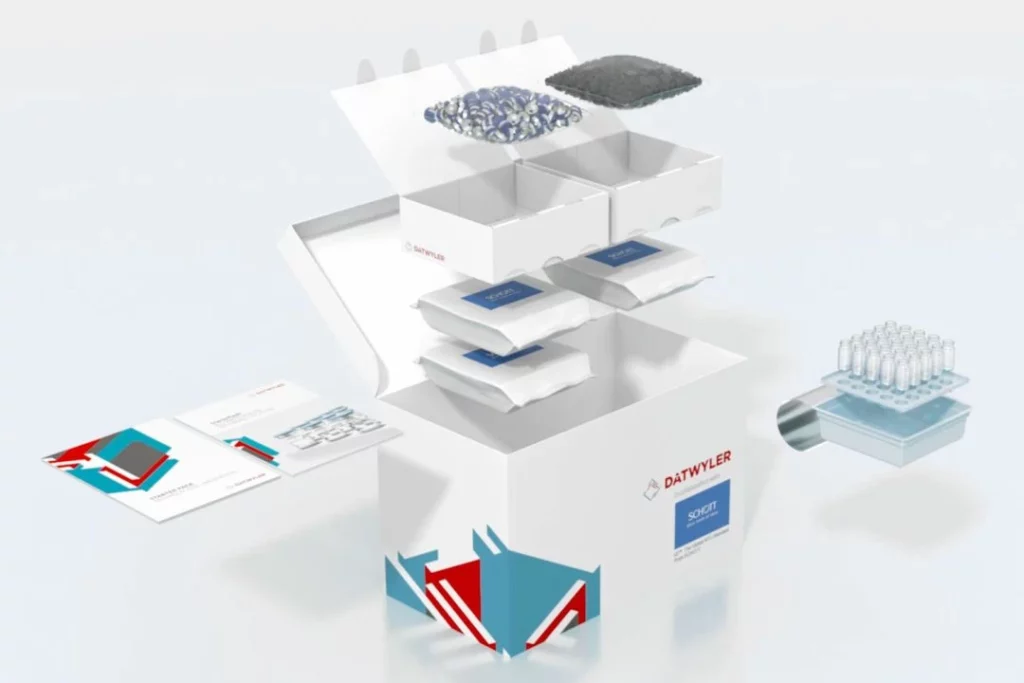
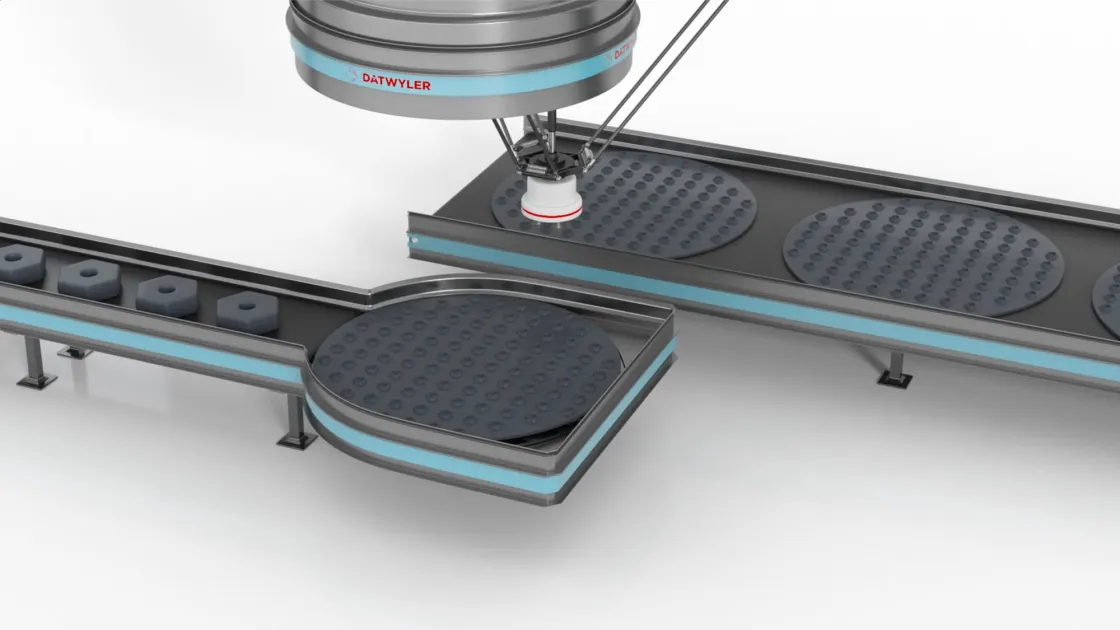
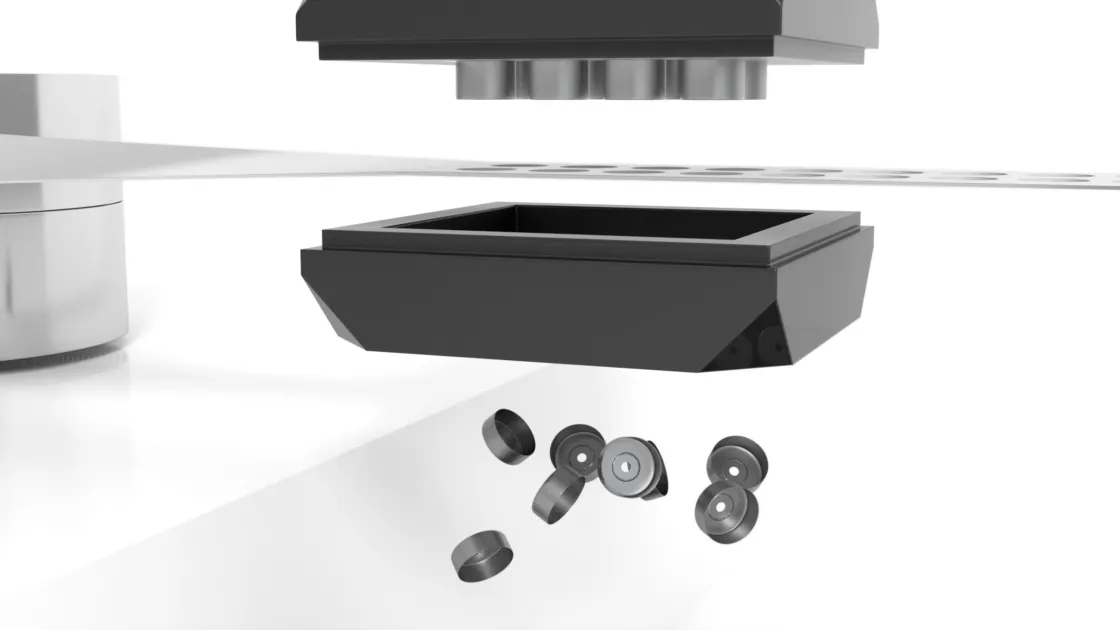
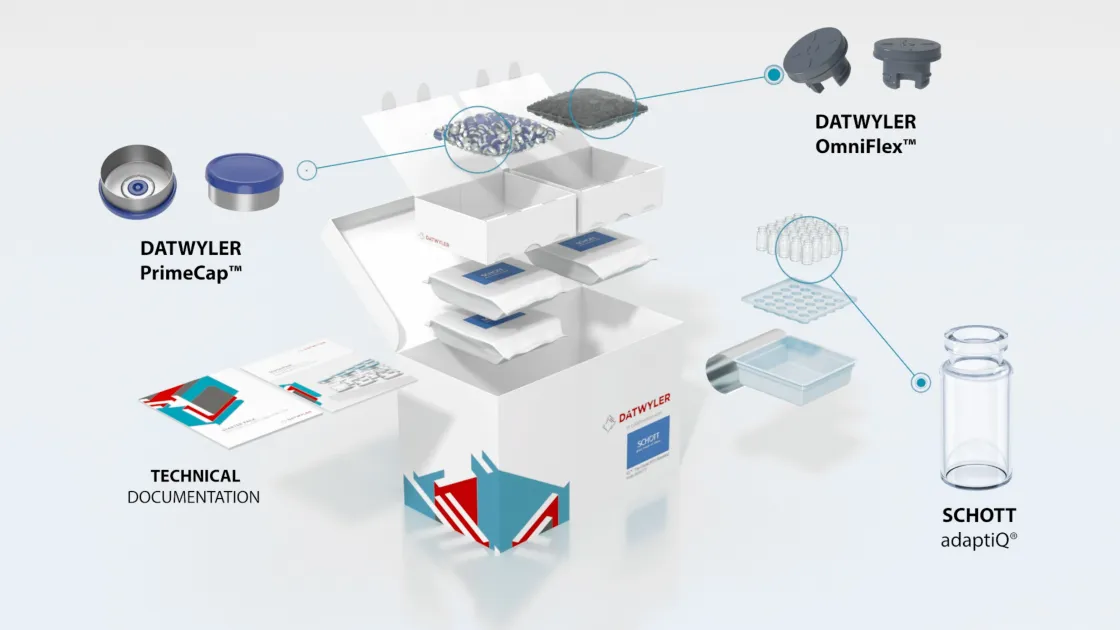
Datwyler has been working with industry leaders to develop sealing solutions for the multiple COVID-19 vaccine candidates. Amid industrywide concerns about shortages, we are in an excellent position to meet projected demand for stoppers and aluminum seals for vaccine packaging. Our team is preparing for increased capacity demands and rapid scale-up in our facilities all over the globe.
The addition of our FirstLine® facility in Middletown, Delaware has provided critical infrastructure to help support the increased demand around COVID-19. By scaling up this facility faster than originally planned and ramping up production with a 24/7 schedule worldwide, we’ve increased capacity for coated components by almost 50% over the last two years and have more investments to optimize the use of our production facilities in the pipeline. These measures not only ensure our readiness for COVID-19, but also enable continued production of sealing solutions for injectable drug packaging used in other medicines.