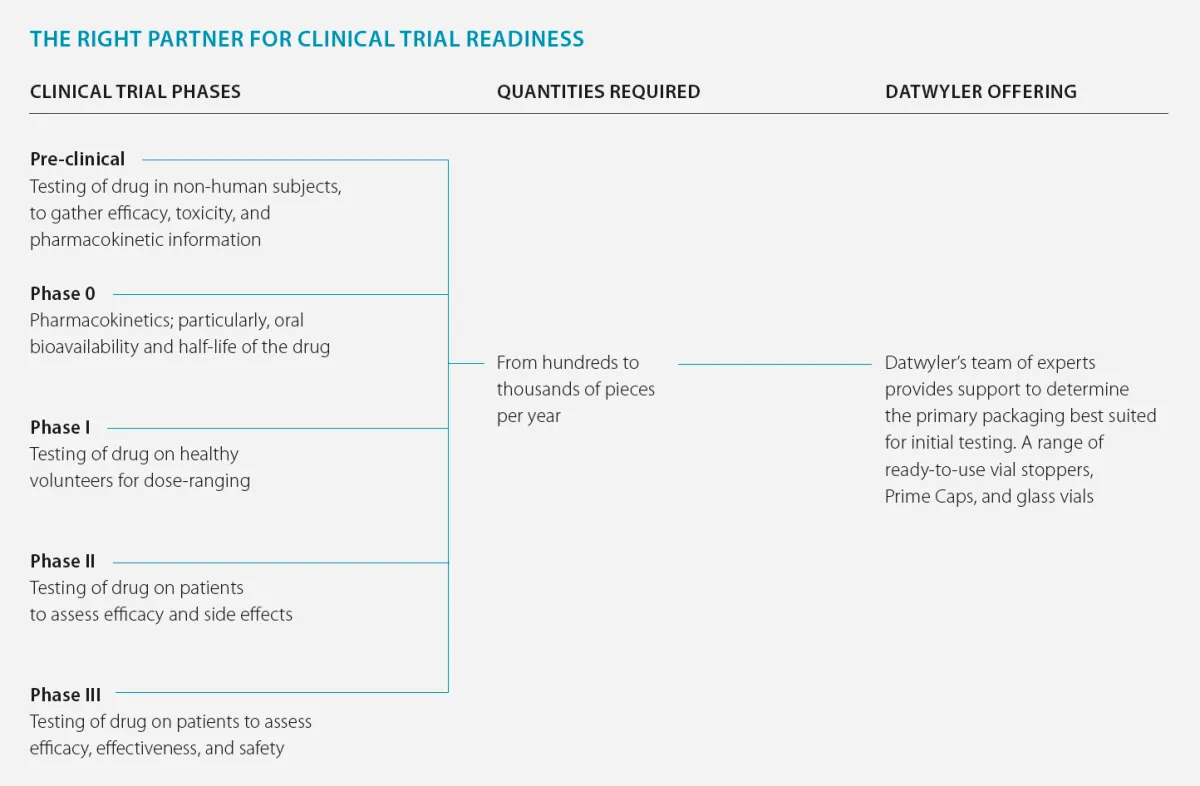
StarterPack
StarterPack™. Datwyler’s solutions for small quantity component needs.
At Datwyler, we are aware of the challenges that customers are facing in their respective fields. Our StarterPack™ is the ideal service to tackle small quantity component needs. We developed the StarterPack™ with SCHOTT to provide our customers with a range of compatible primary and secondary packaging components to be used in every drug development application.
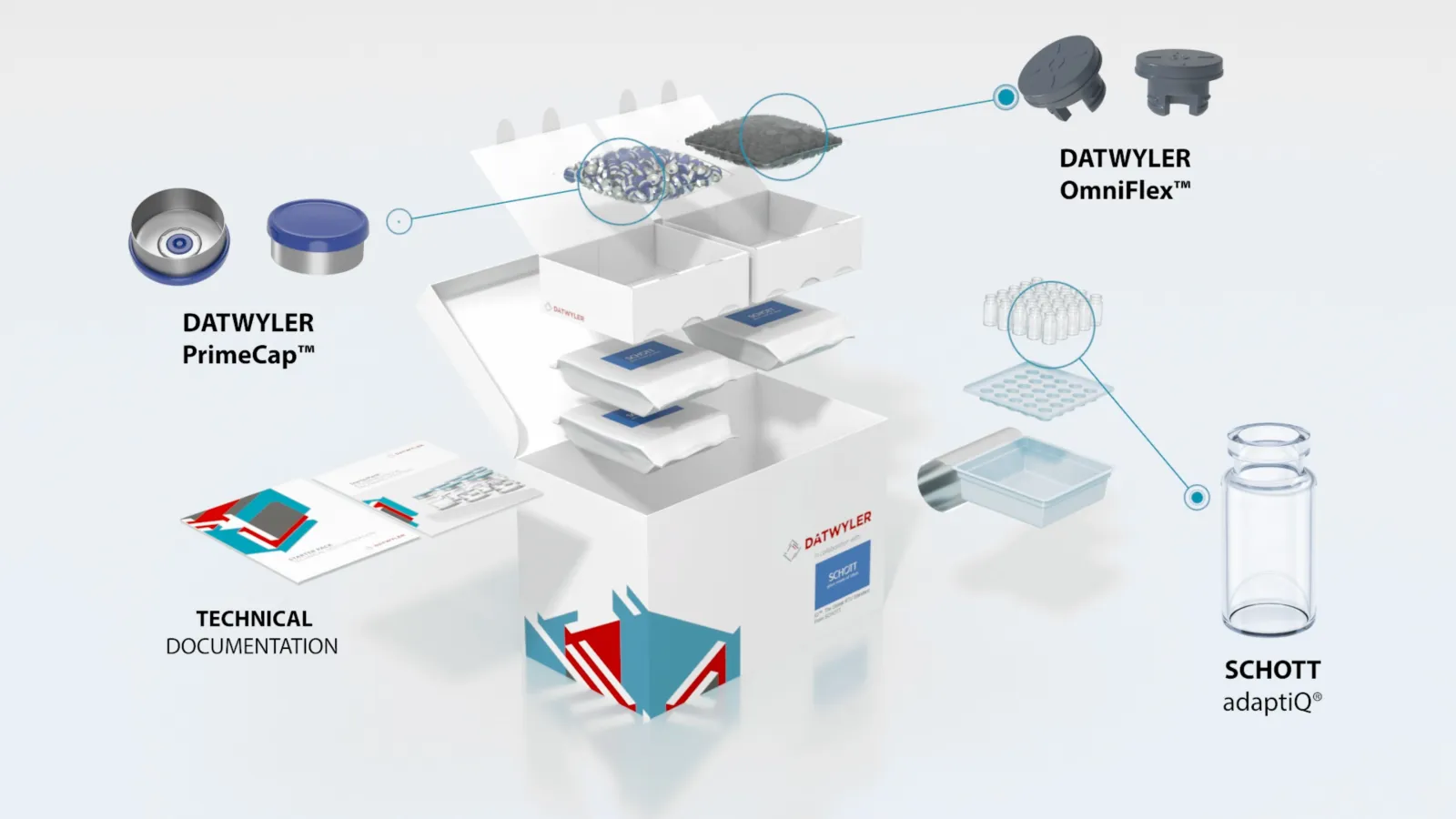
Datwyler StarterPack™
We enable our customers to incorporate high-quality components and commercial packaging solutions into their testing and clinical trial strategy. This includes diagnostic research, clinical trials, as well as, the product launch. Once the drug product is ready for commercial launch, the quantities can be ramped up.
Service offering to meet testing needs.
At Datwler, we offer a high-quality product portfolio, which is completed with a
broad range of services intended to reduce overall costs of ownership. In addition,
we provide analytical and rubber compounding expertise during product selection
and testing.
The implementation of these in-house programs presents our customers with a comprehensive solution for parenteral packaging. Our service offering includes pharmacopoeia and normative testing, functionality testing, Container Closure
Integrity (CCI) testing as well as other relevant testing.
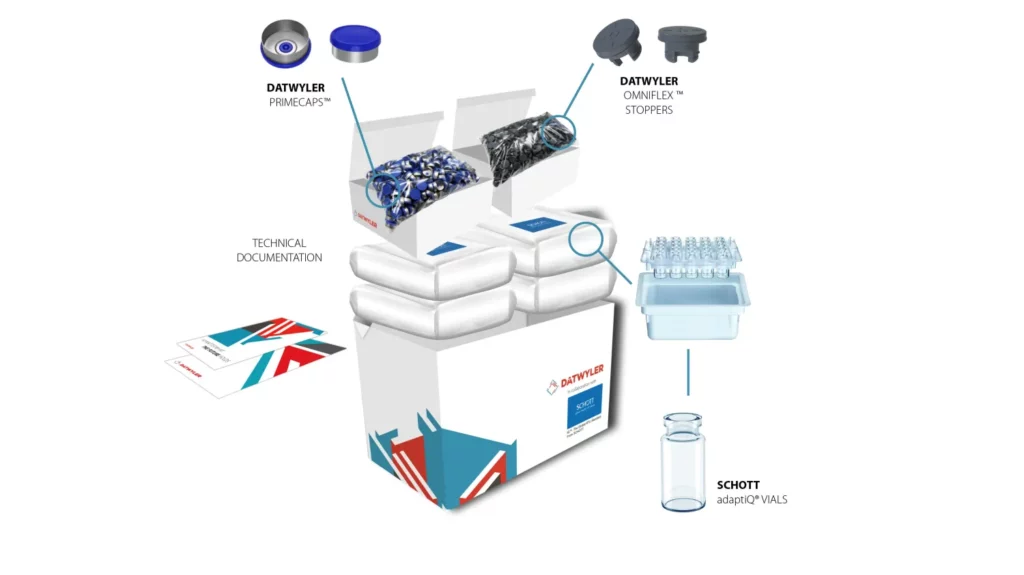
StarterPack™ components.
The Datwyler StarterPack™ includes OmniFlex® Stoppers, PrimeCap™, and SCHOTT adaptiQ® vials. All products provided are ready-to-use (RTU) and have been sterilized in accordance with pharmaceutical and regulatory guidelines.
All of Datwyler’s vial closure solutions are produced, controlled, and tested under the most stringent conditions, enabling the company to meet pharmaceutical requirements to guarantee the highest degree of customer satisfaction and patient safety.
Datwyler’s proprietary fluoropolymer spray coating technology meets the highest demands for quality and performance for highly sensitive, large molecule drugs.
PrimeCap™ are Datwyler’s solution for flawless machineability on high-speed filling lines. All of the company’s facilities worldwide deliver PrimeCap™ in the same quality and with all existing specifications, including the highest quality alloy, two different sizes, center-gated disc for enhanced machineability, low bioburden and particulate levels, and container closure integrity.
The SCHOTT adaptiQ® ready-to-use vials are packaged in an innovative nest with an industry standard tub (SCHOTT iQ® Platform). These can be processed on a wide range of new and existing fill & finish equipment, allowing the vials to remain nested also during lyophilization. The vials are made out of FIOLAX® clear borosilicate glass in TopLine quality for high dimensional and cosmetic quality. adaptiQ® nest design operates completely glass-to-glass contact free and reduces the risk of glass breakage. SCHOTT combines the adaptiQ® nest with an industry standard tub, increasing the flexibility and efficiency for customers. adaptiQ® vials are produced in a cGMP and ISO certified environment, with statistical in-process control and a 100% camera inspection.
The ideal packaging solution.
The Datwyler StarterPack™ is designed to provide our customers with products that guarantee complete CCI. The combination of the Datwyler OmniFlex® stopper, PrimeCap™, and SCHOTT adaptiQ® vials provides an ideal sealing compatibility, preventing leaks and spillage of the product during manufacturing and handling.
Using standard CCI testing methods required by pharmaceutical and regulatory authorities, our team of experts can recommend a total packaging solution for housing our customers’ sensitive drug products.
Resources